Organic reactions follow a logical pathway
involving the atoms and groups of atoms interacting with each other. The route
followed by the reactants to produce products is known as the reaction
mechanism. Organic chemists are usually asked to draw a suitable (plausible)
mechanism for different chemical reactions. There are a few things that need to
be kept in mind while drawing reaction mechanisms correctly, keeping in view
the basic concepts of chemistry in general and organic chemistry in particular.
1. Drawing the reactants and reagents
The
first step for drawing a more probable reaction mechanism is to draw the
reactants and reagents in such a way that the bonds between different atoms in
a molecule are clearly seen and understandable. This helps a lot. For example,
it gives you an idea about the functional groups present in the molecule and
from that the reactivity of these groups towards different reagents or reaction
conditions. Secondly, it helps you find the exact center (atom) that is
involved in the reaction.
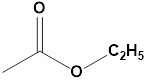
Writing
ethyl acetate as C4H8O2 will not tell you
anything about the reaction centers, but drawing it like
clearly shows the ester group, with the carbonyl carbon and the a-hydrogens, one of which might be the possible reaction center.
clearly shows the ester group, with the carbonyl carbon and the a-hydrogens, one of which might be the possible reaction center.
2. Nature of Reaction (Polar/Non Polar)
Almost
all reactions in organic chemistry (except those involving free radicals)
involve a reaction between an electron rich center and an electron deficient
center. An important step in drawing mechanism is to figure out the nature of
the reaction. If the reaction is of polar nature, it will definitely involve
electron rich and electron deficient centers. These sites can easily be figured
out from the structural formula (given in step-1) and from the background
knowledge of the subject. If the reaction is non-polar, it will involve free
radicals, generated by homolytic cleavage of bonds. Which bonds be cleaved
homolytically, comes from the knowledge of the subject.
3. Reaction Conditions
The
third step to know is the reaction condition. For example, acidic or basic
conditions. The property of an acid is to give H+ in solution, in
other words it provides hydrogen ion for protonation. Base is known for its
electron rich nature and will abstract any acidic proton present in the
molecule, such as the one attached to oxygens, nitrogens in the molecule or the
a-hydrogens in carbonyl
compounds.
If
the reaction is carried out under acidic conditions, the very first thing that
is bound to happen is the protonation of a heteroatom in the molecule, e.g.,
the carbonyl oxygen, oxygen of the alcohol, nitrogen in amines etc.
If
the reaction conditions are basic, an acidic hydrogen is going to be abstracted
first leading to the formation of intermediates after shifting of electrons.
4. Electron Flow Arrows
Drawing
of the electron flow arrows is an important, or probably the most important
thing in drawing reaction mechanisms. The direction of these curved arrows show
the direction of the flow of electrons. Students of organic chemistry sometimes
draw them in a wrong direction.
The
correct way to draw the arrow is to start from an electron rich center and end
at an electron deficient center. This means that electrons are flowing from the
richer center to the deficient center, which is more logical than the other way
round.
TYPES OF ARROWS
These
curved arrows are of different types. If the mechanism is polar there is
usually flow of an electron pair. The arrow drawn in this case is a full headed
arrow. In case of free radical reactions, there is homolytic cleavage involving
the transfer of single electrons, a half headed arrow should be drawn.
5. Which bond to break and make
To
understand which bonds are to be broken and which formed, is very important.
Normally the lone pairs on heteroatoms are more reactive and will react first
to make sigma bonds. Pi bonds are weaker and more reactive than sigma bonds, so
they will react first and are broken. The way they react depends upon the
nature of the reagent and the conditions applied. Charged species are the most
reactive ones, reacting rapidly to form bonds.
6. Balanced Chemical
Equation
Balancing
the equation is necessary as it tells about the molar ratios of the reactants
and the reagents.
An Example:
MECHANISM
Photo credits: www.leah4sci.com









No comments:
Post a Comment